Sabun ve Hijyen
22-06-2023
11:28

Fotoğraf: Bronz Hygieia heykeli, Art Deco formu, Feniks binası köşesi, Ana Çarşı Meydanı, Krakow, Polonya, Heykeltraş: Xawery Dunikowski, Yapım yılı 1929, Mimar: Wladyslaw Klimczak. Feniks binası, 1920'lerin sonlarında eczacılık binası olarak inşa edildi, bundan dolayı, yaklaşık dört metre boyundaki heykel, binanın yapım amacına uygun bir semboldü. Heykel günümüzde Krakow şehrinin sembolüdür. Antik Yunan mitolojilerindeki şifa ve temizlik tanrıçası Hygieianın sol elindeki asaya sarılı yılan şifanın sembolüdür, klasik Yunan heykel sanatındaki tüm Hygieia heykellerinde bulunur. Heykel, sağ elinde, antik Yunan döneminin heykellerinin tersine, su tası yerine taç taşır. Su arınma ve temizlenmenin sembolüdür. Taç, sanat tarihinde kullanıldığı durum ve bağlama göre, birden fazla alanda (müzik, şiir, politika vb.), başarının sembolü olarak kullanılmıştır. Klasik Yunanda, özellikle zeytin dalından yapılan taçlar, galip gelen atletlere zafer ve şeref göstergesi anlamında verilen ödüllerden biriydi. Sanatçı zafer, başarı, sağlık gibi birden fazla kavrama gönderme yapan taç sembolünü kullanmak istemiştir.
"Bir devletin sabun tüketimi sağlık ve medeniyetinin ölçüsü olabilir. Eğer iki ülkenin nüfus toplamları birbirine eşitse, en fazla sağlıklısı ve en fazla medeni olanı daha çok sabun tüketeni olacaktır."
Justus Von Liebig (1803 - 1873), Organik Kimyager, Almanya.
Tanrıça Hygeia ve Hijyen
Hygeia’nın elinde bulunan kase “Hygeia Kasesi” olarak eczacıların sembolüdür. Kasenin içinde bal, zeytinyağı ve buğdaydan oluşan, şifalı bir içecek olduğuna inanılmıştır. Antik Yunan ve Roma dönemi heykellerinde bir elinde yılan diğer elinde kase bulunan kadın figürleri ile tasvir edilir. Tıp bilimi, şifa tanrıçası Hygiea’nın isminden türetilen "hijyen" sözcüğünü temizliği ifade etmek için kullanıyor. Bunun nedeni, kelimenin sadece genel temizliği ifade etmemesidir. Hijyen; temiz koku, tozlardan kurtulma, çıplak gözle görülmeyen ancak var olduğu bilinen, mikroskop yardımı ile görülebilen mikroorganizmalardan arınma durumu demektir, ek olarak; arınmaya yönelik uygulamalarda, bu işlevi gören maddelerin (sabun vb.) kullanılması ile gerçekleştirilen temizlik aksiyonunu ifade eder. Tarihsel süreçte, önceleri tapınaklara girmeden önce gerçekleştirilen kişisel temizlik uygulamaları, insan bilincindeki tarihsel değişimle birlikte, kişisel temizlikten, bireylerin yaşadığı fiziksel çevrenin temizlenmesine kadar genişleyen bir kültüre evrilmiştir.
Anadolu kültüründe ise kasenin yerini "şifâ tasları" almıştır, halk hekimliğinde şifâ tasından su içen insanların hastalıklardan kurtulacağına inanılırdı. Bu tasların geçmişi Arami kavimlerine kadar uzanmaktadır. Orta Anadolu'nun kadim medeniyeti Frigya kazılarında da benzer taslar bulunmuştur.
Sabun Nedir, Nasıl Oluşur?
İnsanlığın en eski tedavi ve temizlik nesnelerinden biri, kimine göre de insanlık tarihindeki en önemli tıbbi buluş olan sabun, yakın zamana kadar toplumların medeniyet ölçüsü olarak kullanılan bir kriterdi. Günümüzde en fazla kullanılan temizlik malzemesidir.
Sabunun temizleyici etkisi, bünyesinde bulunan hidrofobik (sudan kaçınma özelliği) kısmın yağ/kir parçacıklarını sarabilme kapasitesi ile ilişkilidir. Sabundaki bu fonksiyon bir dizi kimyasal reaksiyon sonrasında ortaya çıkar. Buna "sabunlaşma reaksiyonu" adı verilir. Gerçekte en temel kimyasal reaksiyonlardan biridir; asit ile bazın reaksiyona girmesi sonrası tuz oluşumu. Reaksiyon doğal/kimyasal olmayan malzemelerin bir araya gelmesiyle kendiliğinden bile başlayabilir. Örneğin, alkali toprakla karıştırılmış su bir tabuta sızsa, cesette bile sabunlaşma reaksiyonu başlayabileceği bulunmuştur.
Çeşitli yağlara (bitkisel, hayvansal) odun külünden elde edilen kostik (sodyum hidroksit, NaOH) ve su ilavesiyle (bazı şartlarda bir miktar tuz) partiküllü olmakla birlikte, sabunumsu bir bulamaç elde edilebilir. Geçmişte kostik amacıyla kül suyu (ağaç ya da bitki külü) kullanılırdı. Günümüzde, partikülsüz ve pür bir sabun üretmek için saf kimyasallar kullanılmaktadır. Katı bir sabun için sodyum hidroksit, sıvı sabunlar (arap sabunu gibi) için potasyum hidroksit (KOH) kullanılıyor. Yerel sabun üreticileri bu malzeme için "sabuncu sodası" veya "soda" ifadelerini kullanırlar. İki farklı üretim yöntemi mevcuttur; soğuk yöntem ve sıcak yöntem. Soğuk sabun yönteminde elde edilen bulamaç ısıya maruz bırakılmaz, süreç oda ısısında yavaş bir hızda gerçekleşir.
Çeşitli yağlara (bitkisel, hayvansal) odun külünden elde edilen kostik (sodyum hidroksit, NaOH) ve su ilavesiyle (bazı şartlarda bir miktar tuz) partiküllü olmakla birlikte, sabunumsu bir bulamaç elde edilebilir. Geçmişte kostik amacıyla kül suyu (ağaç ya da bitki külü) kullanılırdı. Günümüzde, partikülsüz ve pür bir sabun üretmek için saf kimyasallar kullanılmaktadır. Katı bir sabun için sodyum hidroksit, sıvı sabunlar (arap sabunu gibi) için potasyum hidroksit (KOH) kullanılıyor. Yerel sabun üreticileri bu malzeme için "sabuncu sodası" veya "soda" ifadelerini kullanırlar. İki farklı üretim yöntemi mevcuttur; soğuk yöntem ve sıcak yöntem. Soğuk sabun yönteminde elde edilen bulamaç ısıya maruz bırakılmaz, süreç oda ısısında yavaş bir hızda gerçekleşir.
Üretimdeki en önemli ayrıntı ölçüdür; kullanılan yağın içerdiği yağ asitlerinin cilt üzerine olası istenmeyen etkileri olabilir. Her bir yağı sabunlaştırmak için gerekli alkali oranı farklıdır. Sabunlaşma reaksiyonu için, alkali oranının hassas bir şekilde hesaplanması (sabunlaşma değeri), sabunlaşmamış alkali (serbest alkali) miktarını azaltacak, böylece cildin tahriş olmasının önüne geçecektir. Tarihsel süreçte bu hassasiyette ölçüm yapmak ancak 1800 yıllarının sonunda mümkün oldu, bundan dolayı elde edilen sabunun kalitesi ustanın göz kararına ve tecrübesine bağlıydı.
Sabun kurudukça, yağ ve alkali karışımı fermente olur, hijyenik ve dermatolojik özelliklerini iyileştirir, en az altı ay süren bir işlem olmasına rağmen, bu süreç yıllarca sürebilir. Bundan dolayı, bir sabunun kalitesi, bileşimi ve yaşı ile belirlenir.
Sabun Nasıl İşlev Görür?
Kir ya da pislik olarak adlandırdığımız şeyler cilt, kumaş veya diğer yüzeylere yapışarak, mekanik yöntemler ya da sadece su ile ortamdan uzaklaştıramadığımız, gözle görülen ya da görülemeyen mikroskopik yapılardır.
Sabunun içeriğindeki mölekülün kiri ortamdan uzaklaştırma kabiliyeti, molekülün suyun yüzey gerilimi azaltma kapasitesinden gelir, böylece su yıkanan nesnenin arasına/içine daha kolay nüfuz eder.
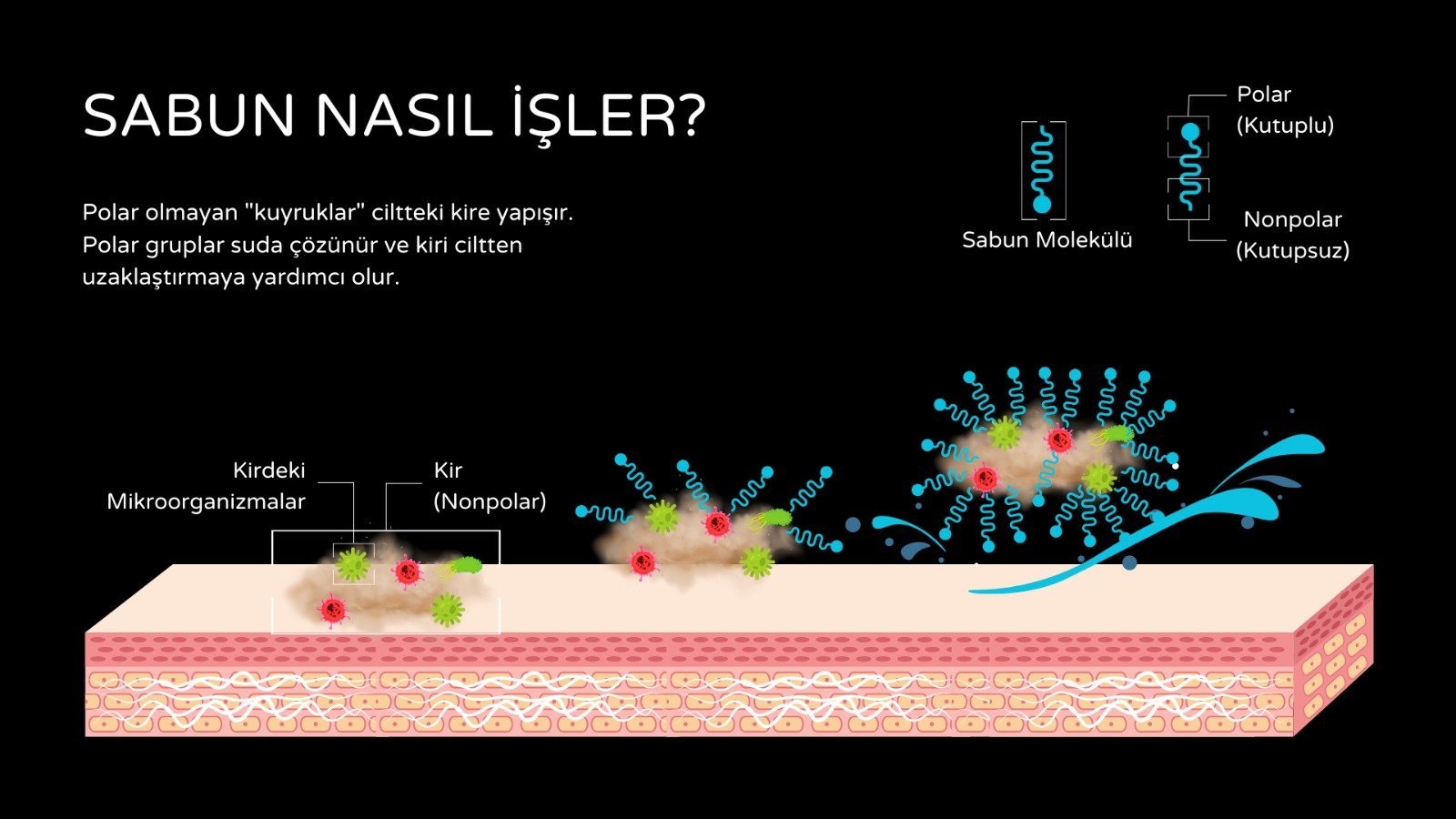

Sabun çözünmeyen kir ve yağ parçacıklarını hapseder, böylece suda çözünebilir iyon kümeleri (misel yapıları) oluşturur. Bu özelliği içeriğinde bulunan uzun zincirli moleküllerden gelir. Bu molekülün bir kutbu hidrofobik (sudan korkan) karakterdeyken diğer kutbu hidrofilik (suyu seven) karakterdedir.
Sabunun içeriğinde bulunan "amfilifiller" adı verilen yağ benzeri moleküller virüsleri etkisiz hale getirir. Molekül virüs duvarında bulunan yağ moleküllerinin arasındaki bağları çözer ve duvar bütünlüğünü bozar. Buna ek olarak, virüsün içindeki protein ve nükleik asitlerden oluşan yapıtaşlarını birbirine bağlayan moleküllerle de rekabete girerek onları parçalayıcı etki gösterir. Böylece hem dış çeperin çözülmesine hem de içerisindeki RNA ya da DNA mölekülünün dağılmasına neden olur. Tüm bunlara ek olarak; virüsün duvarının dış yüzeyinde bulunan, virüsün cilde tutunmasını sağlayan bağlarla da rekabet eder. Bu kapasiteleri açısından virüsleri yok etmek için en etkili seçenektir. Bununla birlikte eğer sabuna erişiminiz yoksa veya ellerinizi sabunla yıkayacak durumda değilseniz alkollü bezler ve sıvı el dezenfaktanları, sabun kadar olmasa da, gene de etkilidirler.
Sabun, virüs dışındaki diğer mikroorganizmaları (bakteriler vb) öldürmez, ama ciltten uzaklaştırır. Su ile birleştiğinde, ortaya çıkan kayganlık, bakterilerin cilde tutunmasını önler, onları yüzeyden koparır, suya karıştırır, durulama ile ortamdan uzaklaşırlar. Bundan dolayı, diğer susuz sterilizasyon jelleri ile kıyaslandığında, mikroorganizmaları uzaklaştırmada, sabun ve su daha etkilidir.
Zeytinyağı Sabunu
Saf zeytinyağından yapılmış sabunlar (Castile veya Marsilya sabunu), diğer yağlardan yapılmışlara nazaran daha yumuşaktır. Zeytinyağında, yağ asitlerinden en fazla oleik asit vardır. Sabun yapımında zeytinyağı kullanımı, olabilecek en düşük oranda sodyum (Na) bağlar, böylece sodyumun tahriş edici etkisi azalır. Deri üzerinde tahriş ve sertleşmeye yol açmaz ve yumuşatıcı etkisi vardır. Cildi nemlendirir, bu açıdan kuru ciltler için faydalıdır.
Geçmişte, doğal ya da saf kalıp sabunlar Anadolu coğrafyasındaki hamam kültürünün özel bir parçası olarak yer almışlardı. Doğada sabun kendiliğinden oluşmaz, o nedenle "doğal sabun" ifadesi yerine "geleneksel sabun" ifadesini kullanmak daha doğrudur.
Binlerce yıldır süregelen el emeğine dayalı sabun üretim zanaatkarlığı ve geleneksel sabun üretim yöntemleri azalarak yok olup gitme aşamasına gelmiştir. Geleneksel üretim yöntemlerinin ustalık gerektiren zahmetli bir iştir, doğal malzemelere erişim güçtür, bu malzemeler maliyetlidir. Tüm bunlara ek olarak; geleneksel yöntemde bilimsel anlamda sabunun içeriğindeki kimyasal miktarının hassas bir ölçüm ile standardize edilememesi "geleneksel sabun" olarak nitelendirilen sabunların üretimini önemli ölçüde geriletmiştir. Endüstriyel sabunların daha az maliyetli ve yaygın olması, geleneksel sabunların pazar paylarının daralmasına neden olmuştur. Kent kimliğinin bir göstergesi olarak "geleneksel sabun" üretim kültürü; ustaları, malzemeleri, üretimin yapıldığı yapıların mimari özellikleri açısından günümüz tarih araştırmalarının bir konusu durumuna geldi.
Kozmetik ve Sabun
Sabun, tarihin en kadim zamanlarından beri içeriğine katılan türlü renk ve koku malzemeleriyle (çiçekler, kökler gibi bitkisel malzemeler, esanslar, kil vb.) sadece tenizlik amacıyla değil, çeşitli cilt problemleri başta olmak üzere bazı hastalıkların tedavisinde de haricen kullanılageldi. Günümüzde de cildi besleyen, cilt sağlığına katkıda bulunan etken maddeleri içeren bitkisel içeriklerin ilavesi hala süregelen bir üretim kültürüdür. Kremler, losyonlar ve benzeri kozmetik ürünlerdeki etken maddeler, vücudumuzun sadece %15-20’si ile buluşurken, sabunlardaki etken bileşenler cildin neredeyse %100’ü ile temas edebilmektedir ve sabunlardaki etken maddeler direkt etki ederler; bundan dolayı, etken maddelerin sahip olduğu sağlığa yararlı özelliklerinden sabun yolu ile daha yüksek düzeyde faydalanırız.
Sonsöz
Sabun temizlik malzemesi olarak asırlardır kullanılıyor. Toplumsal hayatta yaşanan değişim ve dönüşümlere rağmen, özellikle kozmetik ve tuvalet malzemelerinde artan ürün çeşitliliğine karşılık geleneksel sabunlar hala varlıklarını korumaktadır. Günümüz yaşam kültüründe; kimyasallardan uzak durma, hemen her tüketim ürününde "doğal" olana yönelme eğilimi ve doğayla barışık tüketim bilinci yaygınlaşıyor. Belki bu bilinç geleneksel sabun kültürünün yok olup gitmesini yavaşlatabilir.
Kaynaklar:
1. Anadolu Uygarlıklarıda Temizlik Kavramı ve Uygulamalarının Evrimi, Şükran Sevimli, Doktora Tezi, Tez Danışmanı: Prof.Dr.İlter Uzel, T.C. Çukurova Üniversitesi Sağlık Bilimleri Enstitüsü Deontoloji ve Tıp Tarihi A.B.D., 2005.
2. Türk Kültür Coğrafyasından Özel Bir Örnek: Türk Sabunları; Dr. Güven Şahin, İstanbul Üniversitesi Sosyal Bilimler Enstitüsü, Coğrafya Anabilim Dalı, Ağrı İbrahim Çeçen Üniversitesi Sosyal Bilimler Enstitüsü Dergisi, 5/2 Ekim/Oktober 2019 / Ağrı.
3.Her yönüyle sabun, Metin Ertunç, 7/18, https://www.yasamicingida.com/haber/her-yonuyle-sabun/.
5. Türkiye'de Sabunhaneler; Müge Çiftyürek, Doktora Tezi, Sanat Tarihi Anabilim Dalı, Sanat Tarihi Doktora Programı, Pamukkale Üniversitesi, Sosyal Bilimler Enstitüsü, 2021.
6. https://evrimagaci.org/soru/sabun-ellerimizi-nasil-temizler-4977.
7. https://www.nationalgeographic.com/science/article/ashes-to-ashes-soap-to-soap-or-maybe-ashes-to-soap.
7. https://www.nationalgeographic.com/science/article/ashes-to-ashes-soap-to-soap-or-maybe-ashes-to-soap.
